



Molecular imprinting is one attractive biomimetic approach to study/mimic nature. It can be described as a way of making artificial “locks” for “molecular keys”. Following is a brief outline on the imprinting process, which involves four main steps:




Selection Self-Assembly Polymerization Extraction
The resulting MIPs have a permanent memory of the original template in term of complementary size, geometry, and orientation. These specific binding sites can selectively recognize the target molecule, even in a complex solution.
They have high selectivity similar to that of natural systems, therefore, MIPs could be called “artificial antibodies or proteins”.
MIPs are easily obtained by compolyerization of suitable functional monomers and cross-linkers in the presence of the print molecule.
The cost is considerably lower than natural
antibodies or proteins.
These unique properties make them suitable for sensor
technology and have potential applications

The microspheres has been designed to swell and shrink as a function of the changes of their environment, such as temperature, pH, and other analyte, etc. The interaction of analyte and polymer causes polymer to swell or shrink, resulting in a change in the optical properties, such as refractive index (n). Either transmission or reflection measurements can be used to determine analyte concentration.
All of these encourage us to develop a new sensor. First, we design the
reaction to prepare swellable poly NIPA MIP microspheres selectively sensing to
template, then embed them a hydrogel membrane.Microspheres swell at room temperature,
shrink at high temperature. The shrinking extent is different in
different concentration of template. Less shrinking for high concentration of template due to more
interaction of template with polymer, which results in low refractive
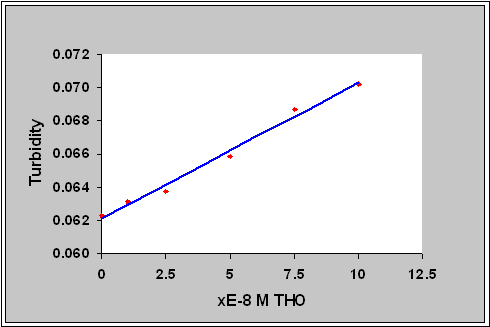
index. So the turbidity changes of the hydrogel membrane could be used to
measure the concentration of template.

Following is the procedure to make theophylline (THO) templated MIPs by dispersion polymerization. THO interacts with MAA via hydrogen bond and electrostatic force. After polymerization, THO can be removed from MIPs by extraction with acetic acid/methanol solution. The resulting imprinted cavity can selectively response to THO. The microspheres are very uniform with the size of ca. 1.0 micron


To exam the molecular specificity, caffeine was selected as
another template molecule with the structure different from THO only by a
methyl group. There is no response to caffeine with the concentration as
high as 1.0x10-4 M.

1x10-8 M THO
Advisor: Dr. W. Rudolf Seitz, Department of Chemistry, University of New Hampshire, Durham, NH 03824